News
Altus Spine Announces FDA Clearance of Sochi OCT System
West Chester, PA – Tuesday, July 8th, 2022 – Altus Spine, a leader in the development and innovation of medical devices used in spinal correction surgery, announces FDA 510(K) clearance of Sochi OCT System. The Altus Spine Sochi OCT System provides a seamless transition from…
Altus Spine heads to Prague, Czech Republic, as Platinum Sponsors of the Society of University Neurosurgeons Annual Meeting!
West Chester, PA – Monday, June 6th, 2022 – Altus Spine, a leader in the development and innovation of medical devices used in spinal correction surgery, announces Platinum Level Sponsorship of the Society of University Neurosurgeons Annual Meeting, being held June 29th-July 3rd, 2022. Located…
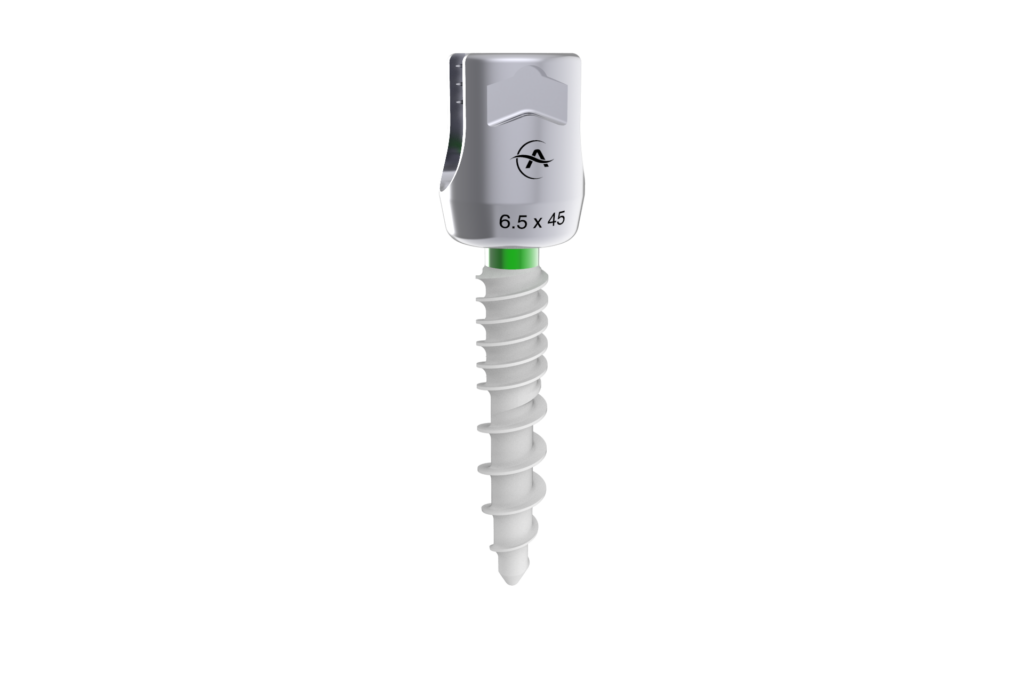
Altus Spine Announces FDA Clearance of Monaco HA Pedicle Screw System
West Chester, PA – May 31, 2022 – Altus Spine, a leader in the development and innovation of medical devices used in spinal correction surgery, announces FDA 510(K) clearance of Monaco HA Pedicle Screw System. Monaco HA Pedicle Screw System, consisting of a low-profile construct…
IEP 2021
Announcement
West Chester, PA – Monday, October 25th, 2021 – Altus Spine, a leader in the development and innovation of medical devices used in spinal correction surgery, announces Foundation Level Sponsorship of the Spine IEP Fellows & Young Surgeons’ Course. The program is taking place in…
NASS 2021 Announcement
Altus Spine is excited to announce our presence at NASS 2021 in Boston! We’ll be meeting with our vendors, clients and friends from around the country. Let us know if you’ll be there!
Interlagos Market Introduction
West Chester, PA- August 30, 2021 – Altus Spine, a leader in the development and innovation of medical devices used in spinal correction surgery, announces the market introduction of the advanced Interlagos MIS Retractor System. The system is a pedicle based lumbar access system designed…
2021 SUN Meeting
Done!
We had a great time attending the Society of University Neurosurgeons (SUNS) annual meeting in Whitefish, Montana. We met academic surgeons from all over the United States, demonstrated our new technologies and learned from the surgeons who visited the booth. Thank you, we sincerely appreciate…
2021 SUN Meeting
Altus Spine is excited to announce our presence at the Society of University Neurosurgeons Annual Meeting being held August 8-11, 2021, in Whitefish, Montana, USA! If you are attending, please come by and visit with us and see what we are all about. thesocietyofuniversityneurosurgeons.com